Oseltamivir phosphate, a prominent antiviral medication, plays a crucial role in combating influenza infections. This drug, commonly known as Tamiflu, effectively targets the influenza virus, hindering its ability to replicate within the body. Its mechanism of action involves inhibiting the neuraminidase enzyme, a crucial protein for the virus to spread and infect new cells. This targeted approach helps reduce the duration and severity of influenza symptoms, offering relief to individuals suffering from the illness.
Oseltamivir phosphate is available in oral form, making it convenient for patients to administer. Its clinical applications extend to both treating existing influenza infections and preventing the virus from taking hold in individuals at risk. However, like any medication, oseltamivir phosphate comes with potential side effects and drug interactions that must be carefully considered.
Oseltamivir Phosphate
Oseltamivir phosphate is an antiviral medication used to treat influenza A and B infections. It is available as an oral capsule or oral suspension and is typically taken twice a day for five days.
Chemical Structure and Properties
Oseltamivir phosphate is a synthetic derivative of the naturally occurring sugar, shikimic acid. Its chemical formula is C16H28N2O6·H3PO4, and it has a molecular weight of 412.4 g/mol. Oseltamivir phosphate is a white, crystalline powder that is soluble in water.
Mechanism of Action
Oseltamivir phosphate is a neuraminidase inhibitor. Neuraminidase is an enzyme that is essential for the release of new influenza viruses from infected cells. When oseltamivir phosphate binds to neuraminidase, it prevents the enzyme from functioning properly, which inhibits the release of new viruses. This ultimately reduces the severity and duration of influenza infections.
Clinical Applications of Oseltamivir Phosphate
Oseltamivir phosphate is a neuraminidase inhibitor that is used to treat and prevent influenza A and B infections. It works by inhibiting the activity of the neuraminidase enzyme, which is essential for the release of new virus particles from infected cells. This inhibition helps to reduce the spread of the virus and shorten the duration of illness.
Indications for Treatment
Oseltamivir phosphate is indicated for the treatment of uncomplicated influenza A and B infections in adults and children who are at least 2 weeks old. It is also indicated for the prevention of influenza in people who are at high risk for complications from influenza, such as the elderly, those with chronic medical conditions, and pregnant women.
Dosage and Administration
The recommended dosage of oseltamivir phosphate varies depending on the age and weight of the patient. It is typically administered orally as capsules or granules for oral suspension. The most common dosage for adults is 75 mg twice daily for 5 days. For children, the dosage is adjusted based on weight.
Effectiveness of Oseltamivir Phosphate
Oseltamivir phosphate has been shown to be effective in reducing the duration of influenza symptoms, particularly when started within 48 hours of symptom onset. However, the effectiveness of oseltamivir phosphate may be limited in some cases, such as when the patient has a severe infection or has been infected with a resistant strain of influenza virus.
Pharmacokinetic Properties of Oseltamivir Phosphate
Oseltamivir phosphate is a prodrug that undergoes rapid and extensive hydrolysis to its active metabolite, oseltamivir carboxylate, after oral administration. This active metabolite exhibits potent antiviral activity against influenza A and B viruses. The pharmacokinetic profile of oseltamivir phosphate encompasses its absorption, distribution, metabolism, and excretion. Understanding these processes is crucial for optimizing its therapeutic efficacy and minimizing potential adverse effects.
Absorption
Oseltamivir phosphate is rapidly absorbed from the gastrointestinal tract following oral administration. Its bioavailability is approximately 75% and is not significantly affected by food intake. The peak plasma concentrations of oseltamivir carboxylate are typically achieved within 2 to 3 hours after oral administration.
Distribution
Oseltamivir carboxylate distributes widely throughout the body, with high concentrations observed in the lungs, where it exerts its antiviral activity. It readily crosses the blood-brain barrier and reaches therapeutic concentrations in the cerebrospinal fluid. The volume of distribution is approximately 20 liters.
Metabolism
Oseltamivir carboxylate undergoes minimal metabolism in the liver. It is primarily excreted unchanged in the urine.
Excretion
Oseltamivir carboxylate is primarily eliminated by renal excretion. The elimination half-life is approximately 10 to 12 hours. Renal impairment can significantly affect the pharmacokinetic profile of oseltamivir phosphate, leading to increased plasma concentrations and potential toxicity.
Factors Influencing Pharmacokinetic Profile
Several factors can influence the pharmacokinetic profile of oseltamivir phosphate, including:
- Renal function: As oseltamivir carboxylate is primarily eliminated by the kidneys, patients with renal impairment may experience increased plasma concentrations and a prolonged elimination half-life. Dose adjustments may be necessary in such cases.
- Age: Pharmacokinetic parameters may differ slightly between adults and children.
- Concomitant medications: Some medications may interact with oseltamivir phosphate, potentially affecting its absorption, metabolism, or excretion.
Comparison with Other Antiviral Medications
Compared to other antiviral medications for influenza, oseltamivir phosphate exhibits a relatively short elimination half-life, typically around 10 to 12 hours. This means that it is cleared from the body more quickly than some other antiviral agents.
For example, zanamivir, another antiviral medication for influenza, has a longer elimination half-life of approximately 17 hours. This difference in elimination half-life may influence the frequency of dosing and the duration of treatment.
Oseltamivir phosphate is also unique in that it is available in an oral formulation, which is convenient for patients. Other antiviral medications for influenza, such as zanamivir, are available as inhaled formulations.
Safety and Side Effects of Oseltamivir Phosphate

Oseltamivir phosphate, a neuraminidase inhibitor, is generally considered safe and well-tolerated. However, like any medication, it can cause adverse effects, some common and others rare. It’s crucial to be aware of these potential side effects and monitor patients closely to ensure safe and effective treatment.
Common Adverse Effects
Common adverse effects associated with oseltamivir phosphate use are generally mild and transient.
- Nausea
- Vomiting
- Diarrhea
- Abdominal pain
- Headache
- Dizziness
- Cough
- Runny nose
- Fatigue
Rare Adverse Effects
Although rare, oseltamivir phosphate can cause more serious adverse effects, requiring prompt medical attention.
- Allergic reactions: These can range from mild skin rashes to severe anaphylaxis, characterized by difficulty breathing, swelling of the face and throat, and a rapid heartbeat.
- Neuropsychiatric events: Some patients, particularly children and adolescents, have reported experiencing hallucinations, delirium, seizures, and changes in behavior after taking oseltamivir phosphate. These events are usually transient and resolve after discontinuing the medication.
- Liver problems: In rare cases, oseltamivir phosphate can cause liver damage, particularly in individuals with pre-existing liver conditions.
- Pancreatitis: Oseltamivir phosphate has been linked to pancreatitis, an inflammation of the pancreas, in rare cases.
Drug Interactions
Oseltamivir phosphate can interact with other medications, potentially affecting their effectiveness or increasing the risk of side effects.
- Probenecid: Probenecid, a medication used to treat gout, can increase the levels of oseltamivir phosphate in the blood, potentially increasing the risk of side effects.
- Drugs metabolized by CYP3A4: Oseltamivir phosphate is metabolized by the enzyme CYP3A4. Medications that inhibit or induce CYP3A4 activity can affect the metabolism and levels of oseltamivir phosphate in the body.
Patient Monitoring
Monitoring patients receiving oseltamivir phosphate treatment is essential to ensure safety and efficacy.
- Monitor for adverse effects: Patients should be closely monitored for any signs or symptoms of adverse effects, especially those listed above.
- Assess liver function: Liver function tests should be performed before starting oseltamivir phosphate treatment and periodically during therapy, particularly in patients with pre-existing liver conditions.
- Monitor for drug interactions: Patients should be questioned about any other medications they are taking, including over-the-counter medications and herbal supplements, to identify potential drug interactions.
Resistance to Oseltamivir Phosphate
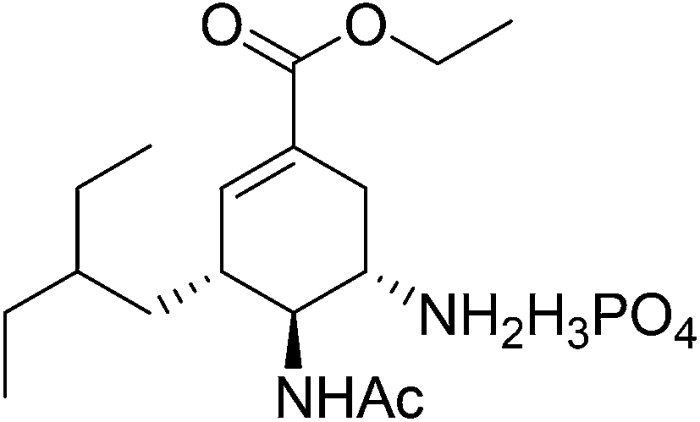
Oseltamivir phosphate, a neuraminidase inhibitor, is a valuable tool in the fight against influenza. However, the emergence of drug-resistant strains poses a significant challenge to its effectiveness. Understanding the mechanisms of resistance and the prevalence of resistant strains is crucial for optimizing oseltamivir’s clinical use and minimizing the selection pressure that drives resistance.
Mechanisms of Viral Resistance to Oseltamivir Phosphate
Viral resistance to oseltamivir phosphate arises primarily from mutations in the neuraminidase (NA) protein, the target of the drug. These mutations alter the active site of the NA enzyme, reducing the affinity of oseltamivir for the enzyme.
- H275Y mutation: This is one of the most common mutations observed in oseltamivir-resistant influenza A viruses. It alters the shape of the active site, making it less hospitable to oseltamivir binding.
- E119V and R292K mutations: These mutations are less common but also contribute to oseltamivir resistance by reducing the binding affinity of the drug.
- N294S and I223V mutations: These mutations are associated with resistance to other neuraminidase inhibitors, such as zanamivir.
Oseltamivir Phosphate in Public Health
Oseltamivir phosphate plays a significant role in public health strategies for managing influenza outbreaks and pandemics. Its effectiveness in reducing influenza severity and duration, along with its potential for prophylaxis, makes it a valuable tool for public health officials.
Role in Influenza Pandemic Preparedness
Oseltamivir phosphate is considered a crucial component of influenza pandemic preparedness plans worldwide. The drug’s ability to shorten the duration of influenza illness and reduce the risk of complications, especially in high-risk individuals, makes it a valuable resource in managing potential pandemic outbreaks.
- Stockpiling: Governments and international organizations stockpile oseltamivir phosphate as a crucial element of their pandemic response strategies. Stockpiles are designed to ensure sufficient supplies are available for widespread use in case of a pandemic.
- Treatment and Prophylaxis: Oseltamivir phosphate can be used both for treating influenza infections and for preventing illness in individuals exposed to the virus. This dual role makes it a versatile tool for pandemic management.
- Antiviral Resistance: The emergence of antiviral resistance poses a significant challenge to influenza pandemic preparedness. Ongoing monitoring and surveillance efforts are crucial to assess the prevalence of resistance and guide public health interventions.
Use for Prophylaxis in High-Risk Populations, Oseltamivir phosphate
Oseltamivir phosphate is recommended for prophylaxis in high-risk populations, including:
- Elderly individuals: Older adults are at increased risk of severe influenza complications.
- Individuals with chronic medical conditions: Individuals with conditions such as asthma, diabetes, heart disease, and kidney disease are more vulnerable to influenza.
- Healthcare workers: Healthcare workers are at high risk of exposure to influenza and can transmit the virus to patients.
- Pregnant women: Pregnant women are more susceptible to severe influenza complications.
Impact on Influenza Morbidity and Mortality
Oseltamivir phosphate has been shown to reduce influenza morbidity and mortality in clinical trials and observational studies. However, its impact on pandemic influenza is less well-defined.
- Reduced Illness Duration: Studies have demonstrated that oseltamivir phosphate can shorten the duration of influenza illness by 1-2 days.
- Reduced Complications: Oseltamivir phosphate can reduce the risk of complications such as pneumonia, bronchitis, and hospitalizations.
- Pandemic Impact: The impact of oseltamivir phosphate on influenza morbidity and mortality during a pandemic is complex and depends on factors such as the severity of the pandemic strain, the timing of treatment, and the availability of other interventions.
Research and Development of Oseltamivir Phosphate Analogues
The continuous emergence of new influenza virus strains, including those resistant to existing antiviral therapies, necessitates the development of novel antiviral agents. Oseltamivir phosphate, a neuraminidase inhibitor, has been a mainstay in influenza treatment, but its effectiveness is threatened by the emergence of resistant strains. Research efforts are actively exploring new avenues to combat influenza, including the development of oseltamivir phosphate analogues.
Potential Advantages and Disadvantages of Alternative Antiviral Therapies
Alternative antiviral therapies offer a promising approach to addressing the challenges posed by influenza virus resistance. These therapies target different viral proteins or pathways, potentially circumventing resistance mechanisms associated with existing drugs like oseltamivir phosphate.
- Advantages:
- Broader Spectrum of Activity: Some alternative antiviral therapies exhibit broader activity against a wider range of influenza virus subtypes, including those resistant to oseltamivir phosphate. This expanded coverage is crucial in combating emerging strains.
- Reduced Resistance Development: By targeting different viral proteins or pathways, alternative therapies may be less susceptible to resistance development, prolonging their effectiveness.
- Improved Pharmacokinetic Properties: Some alternative therapies may exhibit favorable pharmacokinetic properties, such as increased bioavailability or longer half-life, leading to improved therapeutic efficacy.
- Disadvantages:
- Toxicity and Side Effects: New antiviral agents may have different toxicity profiles and side effects compared to existing drugs. Extensive preclinical and clinical testing is crucial to assess safety and tolerability.
- Cost and Accessibility: The development and production of new antiviral agents can be costly, potentially impacting affordability and accessibility, especially in resource-limited settings.
- Limited Clinical Data: The clinical data for many alternative antiviral therapies is still limited, requiring further research to establish their efficacy and safety in real-world settings.
Future Directions for the Development of Oseltamivir Phosphate Analogues
Research into oseltamivir phosphate analogues is ongoing, aiming to improve upon the existing drug’s efficacy and overcome resistance challenges. These efforts focus on several key areas:
- Structure-Activity Relationship Studies: Researchers are investigating the structural modifications of oseltamivir phosphate that can enhance its antiviral activity and reduce resistance development. This involves studying the interactions between the drug and the neuraminidase enzyme, identifying key structural features that contribute to binding affinity and selectivity.
- High-Throughput Screening: High-throughput screening techniques are employed to identify novel neuraminidase inhibitors with improved potency and resistance profiles. This involves testing a vast library of chemical compounds against the neuraminidase enzyme, identifying those with promising inhibitory activity.
- Rational Drug Design: Rational drug design utilizes computational methods to predict and optimize the structure and properties of oseltamivir phosphate analogues. This approach leverages computer simulations and molecular modeling to guide the design of new inhibitors with enhanced efficacy and reduced resistance potential.
- Combination Therapies: Combining oseltamivir phosphate analogues with other antiviral agents or immune modulators could provide synergistic effects and overcome resistance challenges. This approach aims to target different viral proteins or pathways, reducing the likelihood of resistance development.
Ethical Considerations in Oseltamivir Phosphate Use
Oseltamivir phosphate, a neuraminidase inhibitor, has been widely used for the treatment and prevention of influenza. While its effectiveness in reducing the duration of influenza symptoms has been established, its ethical implications have also sparked considerable debate. This section explores the ethical considerations surrounding the use of oseltamivir phosphate, including potential overuse and misuse, the importance of informed consent, and the role of patient education.
Potential for Overuse and Misuse
The widespread use of oseltamivir phosphate has raised concerns about its potential overuse and misuse. The availability of the drug, particularly in the context of influenza pandemics, can lead to increased demand and pressure on healthcare systems. Additionally, the use of oseltamivir phosphate for prevention in healthy individuals without significant risk factors may not be justified, particularly considering the potential for side effects and the emergence of drug resistance.
Overuse of oseltamivir phosphate can contribute to the development of drug resistance, diminishing its effectiveness in future influenza outbreaks.
Furthermore, the use of oseltamivir phosphate in individuals with mild influenza symptoms may not be necessary, as the body’s natural immune response is often sufficient to combat the infection. Misuse of the drug can lead to unnecessary costs, potential side effects, and a false sense of security, potentially hindering public health efforts to control influenza outbreaks.
Informed Consent and Patient Education
Informed consent is crucial for any medical treatment, including the use of oseltamivir phosphate. Patients should be provided with accurate and comprehensive information about the benefits and risks of the drug, including potential side effects, drug interactions, and the possibility of drug resistance. This information should be presented in a clear and understandable manner, allowing patients to make informed decisions about their treatment.
Patients should be informed about the potential benefits and risks of oseltamivir phosphate, including its effectiveness, potential side effects, and the possibility of drug resistance.
Patient education plays a vital role in promoting responsible use of oseltamivir phosphate. Educating patients about influenza prevention strategies, such as vaccination and good hygiene practices, can reduce the need for antiviral treatment and minimize the risk of drug resistance. Additionally, promoting awareness of the appropriate use of oseltamivir phosphate can help prevent its overuse and misuse.
Oseltamivir Phosphate
Oseltamivir phosphate, a neuraminidase inhibitor, has emerged as a crucial antiviral medication for the treatment and prevention of influenza infections. Its discovery and development have significantly impacted global health, particularly in the context of influenza pandemics.
Oseltamivir Phosphate: A Historical Perspective
The development of oseltamivir phosphate is a testament to the relentless pursuit of scientific breakthroughs in the field of antiviral therapy. The journey began in the 1960s with the identification of neuraminidase as a crucial enzyme in the influenza virus life cycle. This discovery paved the way for the development of neuraminidase inhibitors, a class of antiviral drugs that target this enzyme.
- Early Research: In the 1970s, researchers at the Gilead Sciences company embarked on a quest to develop potent neuraminidase inhibitors. They identified a promising lead compound, GS 4071, which exhibited antiviral activity against influenza A and B viruses. However, GS 4071 possessed poor oral bioavailability, limiting its clinical utility.
- The Birth of Oseltamivir: Further research led to the development of oseltamivir, a derivative of GS 4071 with improved pharmacokinetic properties. Oseltamivir exhibited enhanced oral bioavailability and a longer half-life, making it a more suitable candidate for clinical use.
- Clinical Trials and Approval: Extensive clinical trials demonstrated the efficacy and safety of oseltamivir in treating influenza infections. In 1999, oseltamivir phosphate was approved by the US Food and Drug Administration (FDA) for the treatment of uncomplicated influenza in adults and children aged 1 year and older.
The impact of oseltamivir phosphate on the management of influenza infections has been significant.
- Reduced Duration of Symptoms: Clinical studies have shown that oseltamivir phosphate can reduce the duration of influenza symptoms by approximately one day in adults and children.
- Prevention of Influenza: Oseltamivir phosphate has also been shown to be effective in preventing influenza infection in individuals exposed to the virus.
- Pandemic Preparedness: Oseltamivir phosphate has become a crucial component of pandemic preparedness strategies, with stockpiles maintained by governments worldwide to address potential influenza pandemics.
Oseltamivir phosphate has emerged as a valuable tool in the fight against influenza, offering a targeted approach to combatting this prevalent viral infection. Its ability to reduce the severity and duration of influenza symptoms has significantly impacted the management of this illness. However, ongoing research into antiviral resistance and the development of new antiviral agents remains crucial to ensure the continued effectiveness of oseltamivir phosphate and other antiviral therapies in the future.
Oseltamivir phosphate is a medication used to treat influenza, often referred to as the flu. While it’s a crucial tool for fighting off viral infections, it’s important to remember that it doesn’t address other health concerns. For instance, flibanserin, a medication that treats hypoactive sexual desire disorder in women, flibanserin , works on a completely different biological pathway.
Understanding these distinctions is essential for making informed decisions about your health and treatment options. Oseltamivir phosphate remains a valuable tool for treating influenza, but it’s important to seek guidance from a healthcare professional for any health concerns you may have.