Ocrelizumab sets the stage for this compelling narrative, offering readers a glimpse into a story that is rich in detail and brimming with originality from the outset. This medication, a monoclonal antibody, targets a specific protein on immune cells, effectively dampening the immune response and providing relief for patients struggling with autoimmune diseases.
Ocrelizumab has emerged as a transformative treatment option for various autoimmune conditions, demonstrating remarkable efficacy in clinical trials. Its mechanism of action, which selectively targets the immune system, has revolutionized the management of these debilitating diseases, offering hope for improved quality of life for countless individuals.
Ocrelizumab
Ocrelizumab is a monoclonal antibody used to treat relapsing forms of multiple sclerosis (MS) and primary progressive MS. It is a powerful medication that can significantly reduce the frequency and severity of relapses in MS patients.
Mechanism of Action
Ocrelizumab works by targeting and destroying B cells, a type of white blood cell that plays a crucial role in the immune system. In MS, B cells can contribute to the development of inflammation and damage to the myelin sheath, the protective covering around nerve fibers.
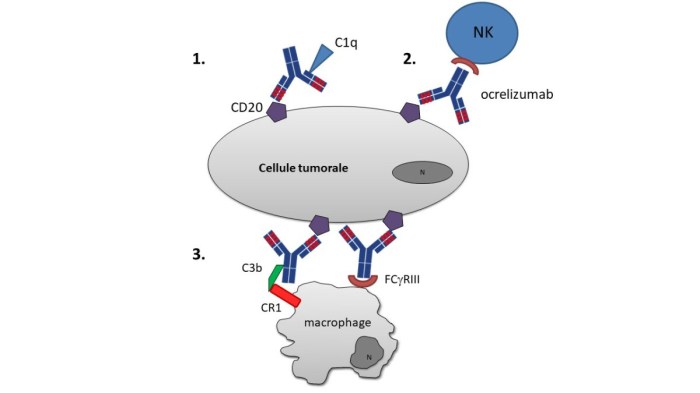
Ocrelizumab specifically targets CD20, a protein found on the surface of mature B cells. When ocrelizumab binds to CD20, it triggers the destruction of B cells through a process called antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC involves the activation of other immune cells, such as natural killer (NK) cells, which then kill the B cells.
By reducing the number of B cells in the body, ocrelizumab helps to suppress the immune system and reduce inflammation in the central nervous system. This can help to slow down the progression of MS and reduce the frequency and severity of relapses.
Chemical Structure and Properties
Ocrelizumab is a humanized monoclonal antibody, meaning that it is primarily derived from human antibodies but with some modifications to improve its effectiveness and safety. It is composed of a heavy chain and a light chain, each containing a variable region that binds to CD20 and a constant region that determines its biological activity.
Ocrelizumab is a glycoprotein, meaning that it contains sugar molecules attached to its protein structure. These sugar molecules play a role in its stability and biological activity. The molecular weight of ocrelizumab is approximately 148 kDa.
Ocrelizumab is administered intravenously as an infusion. It has a long half-life in the body, meaning that it remains effective for an extended period of time.
Therapeutic Applications of Ocrelizumab
Ocrelizumab is a monoclonal antibody specifically designed to target and neutralize CD20, a protein found on the surface of B cells. This targeted action makes it a valuable therapeutic agent in managing certain autoimmune disorders characterized by abnormal B cell activity.
Multiple Sclerosis
Ocrelizumab is approved for the treatment of relapsing-remitting multiple sclerosis (RRMS) and primary progressive multiple sclerosis (PPMS). In RRMS, ocrelizumab effectively reduces the frequency of relapses and slows down the progression of disability. For PPMS, ocrelizumab demonstrates a modest but statistically significant effect in slowing down the progression of disability.
Clinical Trial Evidence
- OPERA I and OPERA II: These pivotal phase III clinical trials evaluated the efficacy and safety of ocrelizumab in patients with RRMS. Results showed that ocrelizumab significantly reduced the annualized relapse rate (ARR) compared to placebo. Additionally, ocrelizumab demonstrated a significant delay in disability progression, as measured by the Expanded Disability Status Scale (EDSS).
- ORATORIO: This phase III clinical trial investigated the efficacy of ocrelizumab in patients with PPMS. While ocrelizumab did not significantly reduce the rate of disability progression compared to placebo, it showed a modest but statistically significant effect in slowing down disability progression.
Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis (ANCA-Associated Vasculitis)
Ocrelizumab is approved for the treatment of ANCA-associated vasculitis (AAV), specifically granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). AAV is a rare but serious autoimmune disorder characterized by inflammation and damage to small blood vessels. Ocrelizumab effectively reduces disease activity and improves remission rates in patients with AAV.
Clinical Trial Evidence
- ADVOCATE: This phase III clinical trial assessed the efficacy and safety of ocrelizumab in patients with GPA and MPA. Results showed that ocrelizumab significantly improved remission rates and reduced the need for corticosteroids compared to placebo.
Pharmacokinetics and Pharmacodynamics of Ocrelizumab
Ocrelizumab is a humanized monoclonal antibody that targets CD20, a protein found on the surface of B cells. Understanding its pharmacokinetic and pharmacodynamic profiles is crucial for optimizing its therapeutic use and managing potential adverse effects.
Pharmacokinetic Profile of Ocrelizumab
The pharmacokinetic profile of ocrelizumab describes how the drug is absorbed, distributed, metabolized, and eliminated from the body. This information is essential for determining the appropriate dosage, frequency of administration, and potential for drug interactions.
- Absorption: Ocrelizumab is administered intravenously, bypassing the process of absorption from the gastrointestinal tract. The drug is infused directly into the bloodstream, ensuring rapid and complete bioavailability.
- Distribution: Once in the bloodstream, ocrelizumab distributes throughout the body, reaching its target sites, primarily the lymph nodes and spleen, where B cells are concentrated. The drug’s distribution volume is approximately 6.5 L, indicating that it distributes widely throughout the body fluids.
- Metabolism: Ocrelizumab is not metabolized in the body. It remains intact and is eliminated primarily through the kidneys.
- Excretion: Ocrelizumab is eliminated from the body mainly through renal excretion, with approximately 80% of the drug being eliminated unchanged in the urine. This process occurs over a prolonged period, resulting in a long half-life of approximately 25 days.
Pharmacodynamic Effects of Ocrelizumab
The pharmacodynamic effects of ocrelizumab relate to its mechanism of action and its impact on the immune system. This antibody selectively targets CD20, a protein expressed on the surface of B cells, leading to their depletion.
- Target Inhibition: Ocrelizumab binds to CD20, blocking its interaction with other molecules and triggering the destruction of B cells. This mechanism of action disrupts the normal development and function of B cells, leading to a reduction in their numbers and activity.
- Immune Modulation: By depleting B cells, ocrelizumab modulates the immune response. This is because B cells are essential for antibody production and the development of humoral immunity. By reducing the number of B cells, ocrelizumab suppresses the production of autoantibodies and other inflammatory mediators, ultimately reducing inflammation and disease activity.
Time Course of Ocrelizumab’s Effects
The time course of ocrelizumab’s effects describes the onset, duration, and potential for accumulation of the drug’s therapeutic effects.
- Onset of Action: Ocrelizumab’s effects typically begin within a few weeks after the first infusion. The drug gradually depletes B cells, leading to a gradual reduction in disease activity. This gradual onset is consistent with the long half-life of the drug and the time required for B cell depletion and subsequent immune modulation.
- Duration of Action: The effects of ocrelizumab persist for several weeks after each infusion. This is due to the drug’s long half-life, which allows for sustained depletion of B cells and continued suppression of the immune response. Regular infusions are required to maintain therapeutic levels and achieve sustained clinical benefit.
- Potential for Accumulation: Due to the long half-life of ocrelizumab, repeated infusions can lead to accumulation of the drug in the body. This accumulation can result in enhanced therapeutic effects but also increases the risk of adverse events. Careful monitoring and adjustment of dosage are crucial to optimize the therapeutic benefit while minimizing potential risks.
Dosage and Administration of Ocrelizumab
Ocrelizumab is administered intravenously or subcutaneously, and the dosage regimen varies depending on the indication and patient factors.
Recommended Dosage Regimen
The recommended dosage regimen for ocrelizumab is as follows:
* Multiple Sclerosis (MS):
* Relapsing-remitting MS (RRMS): 600 mg intravenously over a 3-hour infusion on Day 1 and Day 15, followed by 600 mg every 6 months.
* Primary progressive MS (PPMS): 600 mg intravenously over a 3-hour infusion on Day 1 and Day 15, followed by 600 mg every 6 months.
* Neuromyelitis Optica Spectrum Disorder (NMOSD): 600 mg intravenously over a 3-hour infusion on Day 1 and Day 15, followed by 600 mg every 6 months.
Routes of Administration
Ocrelizumab is available in two formulations:
* Intravenous (IV) formulation: This is the most common route of administration. The IV formulation is administered as a 3-hour infusion.
* Subcutaneous (SC) formulation: This formulation is administered as a single injection into the abdomen, thigh, or upper arm.
Dosage Adjustments
Dosage adjustments may be necessary based on patient factors such as age, weight, and renal function.
* Age: There are no specific dosage adjustments for ocrelizumab based on age.
* Weight: No dosage adjustments are necessary for patients with different weight ranges.
* Renal function: For patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2), the recommended dosage of ocrelizumab is 300 mg every 6 months.
Note: It is important to consult with a healthcare professional for personalized dosage recommendations.
Adverse Effects and Safety Considerations of Ocrelizumab
Ocrelizumab, like many medications, can cause adverse effects. It’s crucial to understand these potential side effects and how they might be managed. This section will discuss common adverse effects, serious adverse events, and potential drug interactions associated with ocrelizumab therapy.
Common Adverse Effects
Common adverse effects of ocrelizumab are generally mild to moderate in severity. They often occur during the initial stages of treatment and may improve over time. These effects are usually manageable with supportive care and rarely lead to discontinuation of therapy.
- Infusion-related reactions: These reactions can occur during or shortly after ocrelizumab infusions. Symptoms may include fever, chills, headache, nausea, and itching. Pre-medication with antihistamines and corticosteroids can help prevent or minimize these reactions.
- Infections: Ocrelizumab can suppress the immune system, making individuals more susceptible to infections. Common infections include upper respiratory tract infections, urinary tract infections, and skin infections. It’s important to monitor for signs of infection and seek medical attention promptly if any develop.
- Gastrointestinal disturbances: Some patients may experience nausea, vomiting, diarrhea, or abdominal pain. These symptoms are usually mild and can be managed with over-the-counter medications.
Serious Adverse Events
While uncommon, serious adverse events can occur with ocrelizumab. These events require immediate medical attention and may necessitate discontinuation of therapy.
- Progressive multifocal leukoencephalopathy (PML): This is a rare but serious brain infection caused by the JC virus. It can lead to neurological complications, including paralysis and death. PML is more likely to occur in patients with a weakened immune system, such as those receiving immunosuppressive therapies. Careful monitoring for signs of PML, such as cognitive decline, weakness, or vision changes, is essential.
- Hepatitis B reactivation: Ocrelizumab can reactivate latent hepatitis B virus in patients who are carriers. This can lead to liver damage and even liver failure. Screening for hepatitis B infection and prophylactic treatment with antiviral medications are recommended for patients at risk.
- Infusion-related reactions: While most infusion reactions are mild, some can be severe and life-threatening. These reactions may involve hypotension, bronchospasm, and anaphylaxis. Careful monitoring during infusions and prompt treatment of any severe reactions are crucial.
- Malignancies: Some studies suggest a potential increased risk of certain malignancies, such as lymphoma, in patients receiving ocrelizumab. However, more research is needed to confirm this association. Regular monitoring for any signs of cancer is recommended.
Drug Interactions
Ocrelizumab can interact with other medications, potentially affecting their efficacy or increasing the risk of adverse effects. It’s essential to inform healthcare providers about all medications, including over-the-counter drugs, supplements, and herbal remedies, being taken before starting ocrelizumab therapy.
- Immunosuppressants: Concomitant use of other immunosuppressants, such as corticosteroids or methotrexate, can increase the risk of infections and other adverse effects. Careful monitoring and dose adjustments may be necessary.
- Live vaccines: Ocrelizumab can interfere with the effectiveness of live vaccines. It’s generally recommended to avoid live vaccines during ocrelizumab therapy and for at least six months after discontinuation.
- Other medications: While specific drug interactions are not well-documented, it’s important to discuss any medications being taken with a healthcare provider to assess potential interactions and adjust therapy as needed.
Monitoring and Management of Ocrelizumab Therapy
Ocrelizumab therapy requires close monitoring to ensure its effectiveness and manage potential adverse effects. This involves regular assessments of the patient’s clinical status, laboratory parameters, and potential complications.
Monitoring Parameters
Regular monitoring is crucial to assess the effectiveness of ocrelizumab therapy and identify potential complications. This includes:
- Disease activity: Monitoring disease activity is essential to assess the effectiveness of ocrelizumab therapy. This can be done through clinical assessments, such as evaluating the patient’s symptoms and functional status, and through laboratory tests, such as measuring the levels of inflammatory markers.
- Infusion reactions: Infusion reactions are a potential complication of ocrelizumab therapy. These reactions can range from mild to severe and can include symptoms such as fever, chills, and hypotension. Patients should be closely monitored during and after infusions for signs of an infusion reaction.
- Infections: Ocrelizumab therapy can increase the risk of infections, including serious infections. Patients should be monitored for signs of infection, such as fever, cough, and difficulty breathing.
- Hepatic function: Ocrelizumab therapy can affect liver function. Liver function tests should be performed before starting therapy and monitored regularly during treatment.
- Complete blood count (CBC): Ocrelizumab therapy can affect the blood count. CBCs should be performed before starting therapy and monitored regularly during treatment.
Management of Adverse Effects
Managing potential adverse effects is a critical aspect of ocrelizumab therapy.
- Infusion reactions: If an infusion reaction occurs, the infusion should be stopped immediately and appropriate medical treatment should be provided. Patients who have experienced infusion reactions may need to receive premedication before future infusions.
- Infections: Patients should be advised to avoid contact with people who are sick and to practice good hygiene. They should also receive vaccinations for preventable infections.
- Hepatic function: If liver function tests are abnormal, the dosage of ocrelizumab may need to be adjusted or the treatment may need to be discontinued.
- Blood count: If the blood count is abnormal, the dosage of ocrelizumab may need to be adjusted or the treatment may need to be discontinued.
Patient Education and Adherence, Ocrelizumab
Patient education is crucial for ensuring adherence to treatment guidelines and achieving optimal outcomes.
- Understanding the disease: Patients should be educated about their disease, including its causes, symptoms, and treatment options.
- Understanding ocrelizumab therapy: Patients should be educated about the benefits and risks of ocrelizumab therapy, including potential adverse effects and how to manage them.
- Adherence to treatment: Patients should be encouraged to adhere to their prescribed treatment regimen, including taking their medication as directed and attending follow-up appointments.
- Reporting adverse effects: Patients should be instructed to report any adverse effects they experience to their healthcare provider.
Ocrelizumab in the Context of Other Treatments
Ocrelizumab is a valuable addition to the therapeutic landscape for multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), but it’s crucial to understand its position relative to other available treatments. Comparing ocrelizumab to alternative therapies helps clinicians make informed decisions about the most appropriate treatment strategy for each patient.
Comparison with Other MS Treatments
Ocrelizumab is a monoclonal antibody that targets CD20, a protein found on B cells, a type of immune cell that plays a role in the development of MS. It is approved for the treatment of relapsing-remitting MS (RRMS) and primary progressive MS (PPMS).
- Disease-Modifying Therapies (DMTs): Ocrelizumab is considered a highly effective DMT, comparable to other high-efficacy DMTs such as alemtuzumab and fingolimod. It has demonstrated efficacy in reducing relapse rates, slowing disability progression, and decreasing brain lesion activity in RRMS. For PPMS, ocrelizumab is the only FDA-approved therapy that has shown a significant effect on slowing disability progression.
- First-line Therapies: Ocrelizumab is often considered a second-line therapy after initial treatment with other DMTs, such as interferon beta or glatiramer acetate, has been unsuccessful or poorly tolerated. However, it may be considered as a first-line therapy for patients with aggressive disease or a high risk of disability progression.
- Other B-cell Depleting Therapies: Other B-cell depleting therapies like rituximab and ofatumumab are also used in MS treatment. While they share a similar mechanism of action with ocrelizumab, they differ in their target antigens and pharmacokinetic properties. Ocrelizumab has demonstrated a more favorable safety profile and a longer duration of action compared to rituximab.
Ocrelizumab in Combination Therapy
While ocrelizumab is generally administered as monotherapy, there are situations where it may be combined with other medications.
- Steroid Treatment: Ocrelizumab may be used in combination with corticosteroids during acute relapses to manage inflammation and reduce symptoms. Steroids are typically administered intravenously or orally for a short duration.
- Other DMTs: Combining ocrelizumab with other DMTs is generally not recommended due to the potential for increased risk of adverse events. However, in specific cases, such as patients with highly active disease, a combination approach may be considered.
Benefits and Limitations of Ocrelizumab
Ocrelizumab offers several benefits over other MS therapies, but it also has limitations.
- Benefits:
- High efficacy in reducing relapse rates and slowing disability progression.
- Long-term efficacy with a convenient dosing schedule (every 6 months).
- Effective in both RRMS and PPMS.
- May offer a better safety profile than other B-cell depleting therapies.
- Limitations:
- Risk of infusion reactions and other adverse events, such as infections and liver enzyme elevations.
- Requires monitoring for potential complications.
- High cost compared to other DMTs.
- Not suitable for all patients, especially those with certain medical conditions or who are pregnant or breastfeeding.
Research and Development of Ocrelizumab
Ocrelizumab’s journey from discovery to becoming a widely used treatment for multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) is a testament to the advancements in biomedical research. Its development involved rigorous preclinical studies and extensive clinical trials, demonstrating its effectiveness and safety profile. Ongoing research continues to explore new applications for ocrelizumab and optimize its therapeutic potential.
History of Ocrelizumab Development
Ocrelizumab’s development traces back to the identification of CD20, a protein found on the surface of B cells, as a potential target for autoimmune diseases. Genentech, a biotechnology company, initiated research on CD20-targeting antibodies, leading to the development of ocrelizumab. Preclinical studies in animal models demonstrated ocrelizumab’s ability to deplete B cells, suggesting its potential therapeutic value.
Preclinical Studies
Preclinical studies, conducted in animal models, played a crucial role in evaluating ocrelizumab’s safety and efficacy before human trials. These studies confirmed ocrelizumab’s ability to deplete B cells, a key mechanism in its therapeutic action.
“Preclinical studies in animal models of autoimmune diseases showed that ocrelizumab effectively reduced disease severity and inflammation.”
Clinical Trials
Ocrelizumab underwent extensive clinical trials, involving thousands of patients with MS and NMOSD. These trials assessed its efficacy, safety, and optimal dosage. The results of these trials demonstrated ocrelizumab’s effectiveness in reducing disease activity, delaying disability progression, and improving quality of life for patients with MS and NMOSD.
“Phase III clinical trials for ocrelizumab in MS and NMOSD showed significant reductions in relapse rates, disability progression, and brain lesion formation.”
Ongoing Research
Research on ocrelizumab continues to explore its potential for treating other autoimmune disorders, including rheumatoid arthritis, systemic lupus erythematosus, and inflammatory bowel disease.
“Ongoing clinical trials are evaluating ocrelizumab’s efficacy in treating other autoimmune disorders, such as rheumatoid arthritis and systemic lupus erythematosus.”
Future Potential of Ocrelizumab
Ocrelizumab’s future potential lies in its ability to target B cells, which play a central role in various autoimmune disorders. Ongoing research is exploring its use in treating other inflammatory and autoimmune conditions, including:
- Systemic lupus erythematosus (SLE): Ocrelizumab’s ability to deplete B cells could potentially be beneficial in managing SLE, an autoimmune disease characterized by inflammation and damage to various organs.
- Rheumatoid arthritis (RA): RA is another autoimmune disease that targets joints, causing pain, stiffness, and inflammation. Ocrelizumab’s B cell depletion could potentially reduce inflammation and improve joint function in RA patients.
- Inflammatory bowel disease (IBD): IBD encompasses a group of disorders that affect the gastrointestinal tract, leading to inflammation and ulcers. Ocrelizumab’s B cell targeting might have therapeutic potential in managing IBD, particularly Crohn’s disease and ulcerative colitis.
Economic and Social Implications of Ocrelizumab
Ocrelizumab, as a groundbreaking treatment for multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD), has profound economic and social implications. Its effectiveness in managing these debilitating conditions comes with considerations related to cost, accessibility, and the impact on patients’ lives and society as a whole.
Cost-Effectiveness of Ocrelizumab Therapy
Assessing the cost-effectiveness of ocrelizumab therapy involves comparing its costs to its benefits, considering alternative treatment options.
- Cost of Treatment: Ocrelizumab is a high-cost medication, requiring significant investment from healthcare systems and individuals.
- Benefits and Outcomes: Studies have demonstrated ocrelizumab’s efficacy in reducing disease progression and improving patient outcomes, leading to fewer hospitalizations and disability.
- Comparison to Alternative Treatments: Cost-effectiveness analyses compare ocrelizumab to other MS and NMOSD therapies, considering factors like drug costs, efficacy, and long-term outcomes.
Economic evaluations often use measures like quality-adjusted life years (QALYs) to quantify the value of treatment. These analyses aim to determine if the benefits of ocrelizumab justify its costs compared to other treatment options.
Impact on Patient Quality of Life and Overall Well-being
Ocrelizumab’s impact on patients’ quality of life is a crucial aspect of its social implications.
- Improved Mobility and Function: By reducing disease progression, ocrelizumab can enhance patients’ mobility, physical function, and independence, improving their daily lives.
- Reduced Fatigue and Cognitive Impairment: Ocrelizumab can alleviate fatigue and cognitive impairment, improving patients’ mental well-being and ability to participate in social activities.
- Enhanced Emotional Well-being: Managing MS and NMOSD can be emotionally challenging. Ocrelizumab’s effectiveness can reduce anxiety and depression associated with these conditions, leading to greater emotional well-being.
The ability to live a more fulfilling and independent life significantly contributes to the overall well-being of patients.
Societal Benefits and Challenges of Widespread Use
The widespread use of ocrelizumab has potential societal benefits and challenges.
- Reduced Healthcare Costs: By preventing disease progression and reducing disability, ocrelizumab can contribute to lower healthcare costs associated with MS and NMOSD management.
- Increased Productivity and Participation: Improved health and well-being can enable patients to participate more actively in the workforce and society, contributing to economic growth and social participation.
- Accessibility and Equity: Ensuring equitable access to ocrelizumab is crucial, considering its high cost and the need for specialized healthcare services.
- Long-Term Impact and Sustainability: The long-term impact of ocrelizumab on healthcare systems and society requires ongoing monitoring and evaluation to ensure its sustainability and effectiveness.
Addressing challenges related to affordability, access, and long-term monitoring is crucial to maximize the societal benefits of ocrelizumab.
Ethical Considerations in Ocrelizumab Use
The use of a biologic therapy like ocrelizumab, while offering potential benefits for patients with multiple sclerosis and neuromyelitis optica spectrum disorder, also raises important ethical considerations. These considerations extend beyond the immediate clinical aspects and encompass broader societal and individual implications.
Informed Consent
Informed consent is a cornerstone of ethical medical practice. It ensures that patients understand the risks and benefits of a treatment before making a decision. In the context of ocrelizumab, patients should be fully informed about the potential risks, including the possibility of serious adverse effects like infections and infusion reactions. They should also be aware of the long-term implications of the treatment, such as the potential for long-term immunosuppression and the need for ongoing monitoring.
Access to Treatment
Access to treatment is a crucial ethical issue, particularly for expensive therapies like ocrelizumab. Factors such as insurance coverage, geographical location, and socioeconomic status can influence a patient’s ability to access this treatment. Ensuring equitable access requires addressing these disparities and implementing strategies that promote fairness and justice.
Equitable Distribution
The allocation of resources, including expensive medications like ocrelizumab, is another ethical concern. It is essential to ensure that the distribution of this therapy is fair and equitable, considering the needs of all patients who could benefit. This involves considering factors such as disease severity, prognosis, and the potential impact on quality of life.
Role of Healthcare Professionals
Healthcare professionals play a vital role in ensuring the ethical use of ocrelizumab. They must be well-informed about the drug’s risks and benefits, and they must engage in open and honest communication with patients. This includes discussing the potential risks and benefits, answering questions, and addressing concerns. Healthcare professionals must also be vigilant in monitoring patients for adverse effects and managing potential complications.
Ocrelizumab represents a significant advancement in the field of autoimmune disease treatment, offering a targeted and effective approach to managing these complex conditions. Its ability to modulate the immune system while minimizing adverse effects has made it a valuable tool for healthcare professionals and a beacon of hope for patients seeking relief from their debilitating symptoms.